UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(D)
of the Securities Exchange Act of 1934
February 26, 2020
Date of report (Date of earliest event reported)
Agile Therapeutics, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-36464 | 23-2936302 |
(State or other jurisdiction of incorporation) |
(Commission File Number) |
(IRS Employer Identification No.) |
|
101 Poor Farm Road Princeton, New Jersey (Address of principal executive offices) |
08540 (Zip Code) |
Registrant’s telephone number, including area code (609) 683-1880
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425). |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12). |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)). |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol(s) | Name of each exchange on which registered |
| Common stock, par value $0.0001 per share | AGRX | The Nasdaq Capital Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter)
Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Item 7.01 Regulation FD Disclosure
On February 26, 2020, Agile Therapeutics, Inc. (the “Company”) updated its corporate presentation that it intends to use at conferences and in meetings with investors. The updates include information on the approval of its first product, its plan for the commercialization of that product and recent financing activities, including the net proceeds from its recent public offering of common stock, which included the exercise in full by the underwriters of their option to purchase additional shares of common stock. The aggregate net proceeds to Agile Therapeutics from the offering were approximately $48.4 million, after deducting underwriting discounts, commissions, and estimated offering expenses. The Company is furnishing herewith a copy of the corporate presentation, which is attached hereto as Exhibit 99.1.
In accordance with General Instructions B.2 and B.6 of Form 8-K, the information included in this Item 7.01 (including Exhibit 99.1 attached hereto), shall not be deemed “filed” for the purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference into any filing made by the Company under the Exchange Act or the Securities Act, except as shall be expressly set forth by specific reference in such a filing.
Item 9.01. Financial Statements and Exhibits.
(d) Exhibits.
| Exhibit Number |
Description | |
| 99.1 | Agile Therapeutics, Inc. Corporate Presentation dated February 26, 2020. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Agile Therapeutics, Inc. | ||
| Dated: February 26, 2020 | By: | /s/ Alfred Altomari |
| Name: | Alfred Altomari | |
| Title: | Chairman and Chief Executive Officer | |
Exhibit 99.1

NASDAQ: AGRX Low - dose, Weekly Contraceptive Patch $3.8B Addressable Market

NASDAQ: AGRX Forward Looking Statement Certain information contained in this presentation and other matters discussed today or answers that may be given in response to questions may include “forward - looking statements” . We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward - looking statements . In particular, the Company’s statements regarding projections and potential future results are examples of such forward - looking statements . The forward - looking statements are subject to important factors, risks and uncertainties, including, but not limited to, risks related to our ability to maintain regulatory approval of Twirla ® , our ability along with our third - party manufacturer, Corium, to complete successfully the scale - up of the commercial manufacturing process for Twirla, including the qualification and validation of equipment related to the expansion of Corium's manufacturing facility, the performance and financial condition of Corium or any of the suppliers to our third - party manufacturer, the ability of Corium to produce commercial supply in quantities and quality sufficient to satisfy market demand for Twirla, our ability to successfully commercialize Twirla, the successful development of our sales and marketing capabilities, the accuracy of our estimates of the potential market for Twirla, regulatory and legislative developments in the United States and foreign countries, our ability to obtain and maintain intellectual property protection for Twirla, our strategy, business plans and focus, and unforeseen market factors or events in our clinical, regulatory and manufacturing development plans ; and other factors, including general economic conditions and regulatory developments, not within the Company’s control . These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements . The forward - looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward - looking statements to reflect subsequent events or circumstance . For additional information about the risks and uncertainties that may affect our business please see the factors discussed in “Risk Factors” in the Company’s periodic reports filed with the SEC . . 2

NASDAQ: AGRX ▪ A champion for healthcare choices women deserve, headquartered in Princeton, NJ ▪ Dedicated to building a robust Women’s Health Franchise ▪ Twirla ® is our first FDA - approved product Weekly Contraceptive Patch $3.7B Addressable Market 3 TWIRLA is indicated as a method of contraception for use in women with a BMI < 30 kg/m 2 for whom a combined hormonal contraceptive is appropriate. Consider TWIRLA’s reduced effectiveness in women with a BMI ≥ 25 to < 30 kg/m 2 before prescribing TWIRLA. TWIRLA is contraindicated in women with a BMI ≥ 30 kg/m 2 .

NASDAQ: AGRX ▪ Short - Term Goal - Establish Agile in the prescription contraceptive market with Twirla, our first FDA - approved product ▪ Long - Term Mission - Broaden our women’s health portfolio, including in areas of unmet medical need 4 Agile’s Corporate Strategy: Become a Leader in Women’s Health Establish Agile in Contraceptive Market with Twirla Become Contraceptive Market Leader Broaden Women’s Health Portfolio in Areas of Unmet Need

NASDAQ: AGRX 5 Agile’s Women’s Health Mission Starts with Contraception Nearly half of pregnancies in U.S. women are unintended 3 Nearly half of unintended pregnancies are due to inconsistent and/or improper use of contraception 4 Women’s individual preferences for contraceptive methods vary and change across their lifetimes as their needs change 5 WHY CONTRACEPTION? WHY DO WOMEN NEED MORE BIRTH CONTROL OPTIONS? Women use contraception for an average of 30 years, and nearly all women use contraception at some point 1,2 Women are more consistent with contraceptive use and stay with a method for longer when using a method of their choosing 4 1 - Hamilton BE, Kirmeyer SE., National Center for Health Statistics. 2017; 2 - Daniels K et al, National Center for Health Statistics. 2013 3 - Finer LB and Zolna MR, NEJM 2016; 4 - Frost JJ and Darroch J., Perspectives on Sexual and Reproductive Health 2008 5 - Mansour D, Int J Women’s Health 2014

NASDAQ: AGRX Potential to reduce burden associated with daily pills 49% of contraception users prefer non - daily method 3 52% are frustrated with taking the pill daily 3 NON - DAILY OPTIONS May be preferred by some women 4 Some women prefer to avoid injections, implants, and intrauterine devices LESS INVASIVE METHODS LOWER ESTROGEN DOSE The dose of estrogen in CHCs is believed to be the primary factor contributing to unwanted side effects 1 The only other non - daily transdermal patch currently available delivers a high dose of estrogen 2 “ Some women are just not good at remembering to take a pill at the same time every day…Others don’t want something in their vagina while others don’t want an injection .” - Ob/Gyn 1 - Poindexter, A., Fertility and Sterility 2001; 2 - Xulane Package Insert; 3 - Mansour D., International Journal of Women’s Health 2014; 4 - Qualitative and quantitative HCP research, Kantar Health 2010; Third party research, 2017 6 What is Missing From Available Hormonal Birth Control Options?

NASDAQ: AGRX 30 µg/day Ethinyl Estradiol (EE) 120µg/day Levonorgestrel (LNG) 7 Twirla Designed to Fill A Hormonal Birth Control Market Need Less invasive than some methods (vaginal ring, IUDs, injections, implants) “I want to eliminate the forgetfulness… but I don’t want to lose that control either.” – Consumer, October 2016 Source: Qualitative consumer market research, Adelphi Research 2016 Pill Regimen: Once - a - day Patch Regimen: Once - a - week NON - DAILY LESS INVASIVE HORMONE PROFILE

NASDAQ: AGRX 8 U.S. Hormonal Contraceptive Market is a Significant Opportunity US Market Estimates (2018) $1.6B $5.3 Billion U.S. Contraceptive Market Combined Hormonal Contraception (CHC) Progestin - Only (P - Only) Long Acting Reversible Contraception (LARC) CHC Pill, Ring, Patch P - only Pill, Injection IUD, Implant $3.7 Billion $300 Million $1.3 Billion CHC Pills + Ring + Patch = $3.7 Billion Potential Addressable Market for Rings (18%) Patches (5%) IUDs (16%) Implants (8%) Injectables (5%) P - Only Pills (1%) CHC Pills (48%) P - only Pills category includes emergency contraceptive prescriptions. Not Shown: “All Other” category with <1M TRx Sources: IQVIA NSP through Dec 2018; ACOG FAQs

NASDAQ: AGRX 9 Twirla has the Potential for Significant Market Share Peak TRx Share Estimate Based on Consumer & Physician Market Research and Market Analogs Sources: IMS NPA, 2002 - 2014 Qualitative and Quantitative HCP and Consumer market research, Adelphi Research 2016 Quantitative HCP market research, MarketVision Research 2019 5 - 8% * HCP Market Research (% CHC Market TRx) Study Year Stated Share Calibrated for Overstatement 2019 20% 14% 2016 23% 14% Average of Analog Brands 9.6% Consumers “Extremely Likely” to Ask for Twirla 15% * Will continue to analyze market and update market research based on approved labeling

NASDAQ: AGRX 10 Recent Regulatory History Dec. 2017 Complete Response Letter (CRL) from FDA June 2018 Formal Dispute Resolution (FDR) on Adhesion Initiated Oct. 2018 Resolution of FDR & Potential Path Forward for Resubmission 2018 2020 Feb. 2019 Completion of Comparative Wear Study – Primary Endpoint Achieved 2019 Oct 2019 FDA Advisory Committee Meeting Votes 14 - 1 - 1 in Favor of Approval May 2019 New Drug Application (NDA) Submitted & Accepted PDUFA GOAL DATE: February 16, 2020

NASDAQ: AGRX CLARIFICATION FROM FDA FOR NEXT STEPS New method appears reasonable and will be a review issue Responses to initial PAI submitted. Likely subject to another PAI FDR completed; FDA recommended Comparative Wear Study with Xulane ® FDA anticipates reviewing efficacy and benefit/risk at Advisory Committee 11 The Path To Potential Twirla Approval ISSUE RAISED BY FDA CRL Adhesion Test Methods Manufacturing Inspection Observations In Vivo Adhesion High Pearl Index Achieved Primary Endpoint in Comparative Wear Study & Demonstrate Non - Inferior Adhesion to Xulane ® Completed NDA Submission to FDA Completed PAI at Corium Favorable FDA Advisory Committee on Efficacy and Benefit/Risk REGULATORY MILESTONES ACHIEVED TO DATE PDUFA GOAL DATE: February 16, 2020

NASDAQ: AGRX MARKET ACCESS ▪ Agile knows its market ▪ Top 8 payers expected to cover majority of commercial lives 1 ▪ Strategic contracting inteded to place Twirla in competitive reimbursement position SALES FORCE ▪ Small, targeted sales force (70 - 100 reps) to launch ▪ Phased hiring linked to formulary acceptance ▪ Focus on high - prescribing Ob/ Gyns and women’s health NP/PAs MANUFACTURING ▪ Corium is an experienced contract patch manufacturer ▪ Qualification of commercial scale equipment in final stages ▪ Plan for completion of validation in fourth quarter LAUNCH NETWORK ▪ Relationships with experienced vendors to facilitate commercial launch ▪ Vendors have expertise in marketing, PR, market access, and supply chain Sources 1 - Berchick E, Hood E, Barnett J,. Health Insurance Coverage in the United State 2017 Haefner, M. America's largest health insurers in 2018 12 Agile Has Activated Partners to Prepare for Twirla Commercial Readiness

NASDAQ: AGRX Initiate consumer campaign 13 Phased Approach to Commercial Strategy Pre - PDUFA PRE - MARKET PREP Set up trade distribution Plan payer strategy Marketing Analytics & Strategy Launch manufacturing Payer strategy Formulary access Hire & train sales force Launch sales force Implement HCP Campaign Samples Availability PRODUCT ACCESS HCP ENGAGEMENT CONSUMER ACTIVATION Pre - Validation Validation Commercial Launch Manufacturing Preparations FDA post marketing requirement and commitment: • Long - term prospective, observational post - marketing study comparing risks of venous thromboembolism (VTE) and arterial thromboem bolism (ATE) in new users of Twirla to new users of other CHCs • Small residual drug study to analyze EE and LNG content after prescribed wear and to monitor adhesion

NASDAQ: AGRX 0 10 20 30 40 50 60 70 ESI CVS OptumRx UHC Anthem Aetna Kaiser Cigna HCSC MedImpact Prime Thx Humana BCBS-MI BCBS-CA BCBS-FL Highmark Carefirst Emblem BCBS-MA HealthNet Top 20 Commercial Payers (MM Lives) PBM National Insurer Regional Insurer Managed Care Strategy: Minimize Access Barriers 14 ~ 75% of Commercial Lives Sources 1 - Berchick E, Hood E, Barnett J,. Health Insurance Coverage in the United State 2017 Haefner, M. America's largest health insurers in 2018 Strategy Will Inform Sales Force Rollout Plan Twirla ® Managed Care Strategy Patch Replacement Program Pricing Strategy Affordable Care Act State Mandates

NASDAQ: AGRX $35.0 Million debt facility signed February 10, 2020 ▪ $20 million disbursed ▪ $5 million in proceeds at signing ▪ $15 million in proceeds at Twirla ® approval ▪ $15 million upon $20 million TTM revenue milestone 15 AGRX Financial Overview Cash Balances ▪ $34.5 million in cash and cash equivalents as of December 31, 2019 $ 48.4 Million estimated net proceeds from public offering of common stock ▪ 17.3 million shares sold including underwriters exercise of option ▪ 87.1 million estimated common shares outstanding at February 25, 2020 FEB. 2020 PUBLIC OFFERING BALANCE SHEET FEB. 2020 PERCEPTIVE DEBT FACILITY
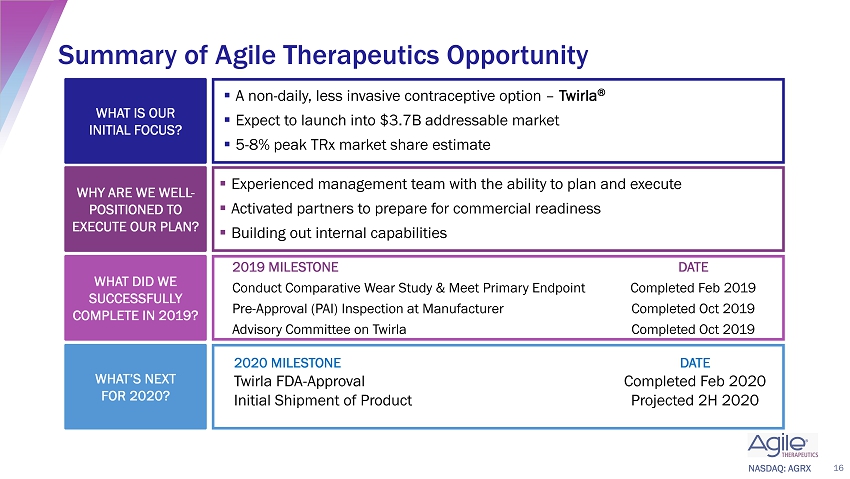
NASDAQ: AGRX WHAT IS OUR INITIAL FOCUS? ▪ A non - daily, less invasive contraceptive option – Twirla ® ▪ Expect to launch into $3.7B addressable market ▪ 5 - 8% peak TRx market share estimate WHY ARE WE WELL - POSITIONED TO EXECUTE OUR PLAN? ▪ Experienced management team with the ability to plan and execute ▪ Activated partners to prepare for commercial readiness ▪ Building out internal capabilities WHAT DID WE SUCCESSFULLY COMPLETE IN 2019? 16 Summary of Agile Therapeutics Opportunity 2019 MILESTONE DATE Conduct Comparative Wear Study & Meet Primary Endpoint Completed Feb 2019 Pre - Approval (PAI) Inspection at Manufacturer Completed Oct 2019 Advisory Committee on Twirla Completed Oct 2019 WHAT’S NEXT FOR 2020? 2020 MILESTONE DATE Twirla FDA - Approval Completed Feb 2020 Initial Shipment of Product Projected 2H 2020

NASDAQ: AGRX 17 Appendix

NASDAQ: AGRX Contraceptive Use by U.S. Women 18 Any method 37.8M No method, at risk 4.4M No method, not at risk 19.3M Contraceptive Method Choice (Number of U.S. Women*) Number of U.S. women using a CHC method* (pill, ring, patch) 10,547,479 *In 2014 Source: Fact Sheet - Contraceptive Use In the United States, Guttmacher Institute, July 2018

NASDAQ: AGRX 19 Average Price Per Cycle for Branded CHCs ($WAC)
