Agile Therapeutics, Inc. Announces that Twirla® Meets Primary Endpoint in Comparative Wear Study and Demonstrates Non-Inferior Adhesion to Xulane®
Company now focused on completing plan to resubmit NDA in first half of 2019
|
|||||
PRINCETON, N.J.,
The Company conducted the comparative wear study as part of its plan to implement the recommendations of the FDA’s
The comparative wear study design follows the 2018 ANDA Guidance for Assessment of Adhesion entitled Assessing Adhesion With Transdermal and Topical Delivery Systems for ANDAs. The study was a randomized, open-label, crossover adhesion study in healthy women aged 18 to 35 years with a Body Mass Index of less than 35 kg/m2. Subjects were randomized to wear either Twirla or Xulane for the first week and then switched to the patch not initially worn for the second week. Eighty-three subjects were randomized; 79 subjects completed the study, and 77 subjects were included in the Per Protocol population used in the primary analysis. Investigators assessed patch adhesion for each day of wear and assigned the patch a daily score ranging from 0 (essentially no patch lift off skin) to 4 (complete patch detachment).
The primary endpoint for the study was the mean difference in adhesion scores between Twirla and Xulane. As agreed upon at the
Table 1. Primary endpoint: mean adhesion scores for Twirla and Xulane
| Twirla | Xulane | Difference (Twirla – Xulane) | |||||||
| N |
Mean (SD) |
N |
Mean (SD) |
Mean (SD) |
One-sided upper 95% CL |
Non-inferiority criterion met |
|||
| Adhesion score in the Per Protocol population | 77 | 0.14 (0.28) | 77 | 0.39 (0.40) | -0.25 (0.23) | -0.16 | Yes | ||
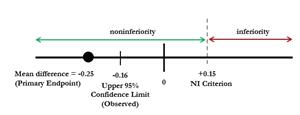
Table 1 shows the results for the primary endpoint: the mean difference in adhesion scores between Twirla and Xulane. The mean difference in Twirla minus Xulane is -0.25. The mean adhesion score for Xulane is higher than the mean score for Twirla, producing a negative mean difference. The upper bound of the 95% confidence limit for the mean difference is -0.16, thus Twirla met the non-inferiority criterion of +0.15. (See Figure 1).
Figure 1. Non-inferiority (NI) scale
A graphic accompanying this announcement is available at http://www.globenewswire.com/NewsRoom/AttachmentNg/eb520acd-e1fe-4038-8798-fce04909d5c0.
No complete detachments of Twirla or Xulane occurred during the trial. The final study report, when complete, will contain additional analyses pertaining to secondary endpoints and safety data.
“We believe that the topline data from our comparative wear study provide important insights into the adhesion performance of Twirla that we can share with the FDA to support that Twirla demonstrates adequate in vivo adhesion. While the results from the study will need to be reviewed by the FDA as part of our planned NDA resubmission, we are very pleased with the results,” said Dr. Elizabeth Garner, Senior Vice-President and Chief Medical Officer of Agile. “We greatly appreciate the hard work and dedication from our clinical team and wish to thank the research professionals and staff at
The Company plans to include the results of the comparative wear study along with additional information relating to the manufacture of Twirla in its response to the Complete Response Letter (“CRL”) it received in December 2017. The FDA has previously informed the Company that in connection with its review of the Twirla NDA, the FDA plans to bring the safety and efficacy of Twirla to an
“We believe that the topline results from the comparative wear study enable us to respond to the in vivo adhesion questions raised by the FDA in the
About Twirla® (AG200-15)
Twirla (levonorgestrel/ethinyl estradiol transdermal system) or AG200-15 is an investigational low-dose, once-weekly contraceptive patch. AG200-15 is a combined hormonal contraceptive (“CHC”) patch that contains the active ingredients ethinyl estradiol (EE), a type of estrogen and levonorgestrel (“LNG”), a type of progestin. Twirla is designed to be applied once weekly for three weeks, followed by a week without a patch. The Company has completed its Phase 3 clinical trials of Twirla and is pursuing regulatory approval in the U.S. Agile received a complete response letter (“CRL”) from the FDA in
About
Agile Therapeutics is a forward-thinking women's healthcare company dedicated to fulfilling the unmet health needs of today's women. Our product candidates are designed to provide women with contraceptive options that offer freedom from taking a daily pill, without committing to a longer-acting method. Our lead product candidate, Twirla® (levonorgestrel/ethinyl estradiol transdermal system), also known as AG200-15, is an investigational low-dose, non-daily, prescription contraceptive. Twirla is based on our proprietary transdermal patch technology, called Skinfusion®, which is designed to allow drug delivery through the skin. For more information, please visit the company website at www.agiletherapeutics.com. The Company may occasionally disseminate material, nonpublic information on the Company’s website.
Follow Agile on Linked In and Twitter: @AgileTher.
Xulane® is a registered trademark of
Forward-Looking Statements
Certain information contained in this press release includes "forward-looking statements," within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, related to our regulatory submissions. We may, in some cases use terms such as "predicts," "believes," "potential," "continue," "anticipates," "estimates," "expects," "plans," "intends," "may," "could," “might," “likely,” "will," "should" or other words that convey uncertainty of the future events or outcomes to identify these forward-looking statements. Our forward-looking statements are based on current beliefs and expectations of our management team that involve risks, potential changes in circumstances, assumptions, and uncertainties, including statements regarding our belief that a reformulation of Twirla may not be necessary. Any or all of the forward-looking statements may turn out to be wrong or be affected by inaccurate assumptions we might make, or by known or unknown risks and uncertainties. These forward-looking statements are subject to risks and uncertainties including risks related to our ability to manage costs and execute on our operational and budget plans, the FDA requiring us to reformulate Twirla, our ability to develop a reformulation that will address the FDA’s concerns, including showing bioequivalence, if necessary, that the results of the comparative wear study do not support a conclusion by the FDA that Twirla has demonstrated adequate adhesion, the potential that we may be required to conduct an additional Phase 3 trial, the likelihood that we will require additional correspondence with the FDA prior to the resubmission of our NDA, our third-party manufacturer’s ability to successfully complete a pre-approval inspection, our ability to resubmit and the timing of our resubmission of the NDA for Twirla, FDA acceptance and approval of the resubmitted NDA, or whether other issues will arise that will negatively impact acceptance, review, and approval of Twirla by the FDA, including a determination by the
SOURCE:
Contact:
Investor Relations
Agile Therapeutics
609-683-1880

Source: Agile Therapeutics, Inc.